The amide shown here, and in Fig 2, is the primary amide from ethanoic acid (acetic acid); the amide is called ethanamide (acetamide).
This page discusses some features of amides that are covered inadequately in some introductory books. I think the points made here should be part of even an introduction to amides.
Everything on this page is reasonably covered in the Ouellette textbook (Organic Chemistry - A Brief Introduction, 2/e, 1998), pp 392, 401 & 436. Thus this page is now a secondary supplement, for my Intro Organic/Biochem with Ouellette. Students in my class using Ouellette are responsible for the material at the level in the textbook; this page may be a useful supplement, but it is not essential.
This page was originally written for use with the Bettelheim textbook (Introduction to General, Organic & Biochemistry, 6/e, 2001). In that book, these issues are either not mentioned or not explained.
This page may also be of interest to students in Molecular Biology, relevant to protein structure.
Bottom of page; return links and contact information
The amide linkage has some interesting properties that may not be obvious from earlier material. Introductory books such as the previous X402 book vary in how much they present or explain these properties; unfortunately, they often leave these properties as mysteries to be memorized. This page serves as a brief explanation of some properties of the amide linkage.
A couple of "surprising" properties of amides that one learns about are:
Amides are neutral compounds -- in contrast to their seemingly close relatives, the amines, which are basic. (Bettelheim may not mention this at all. It can be inferred by omission, since the book does mention acid and base properties when relevant.)
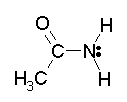
The amide linkage is planar -- even though we normally show the C-N connected by a single bond, which should provide free rotation. (Bettelheim shows the planarity of the peptide bond, a specific example of amide bonds, on p 506.) Fig 1 below shows this common drawing of an amide.

|
Fig 1. An amide; usual representation.
The amide shown here, and in Fig 2, is the primary amide from ethanoic acid (acetic acid); the amide is called ethanamide (acetamide). |
To help understand these properties, we need to look at a more complex -- but better -- representation of the amide structure. This is shown in Fig 2:
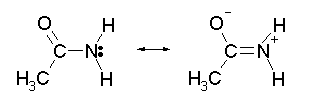
|
Fig 2. Resonance structures for an amide.
Remember that the molecule does not actually switch between these structures. Instead, the actual structure is somewhere in between the structures shown. It can be thought of as some average of these structures. |
Why is this resonance system better? A qualitative argument is that the O, which is very electronegative, draws electrons toward it. In this case, it draws electrons from the lone pair of the N. Note that in the right hand form, the electrons of the N lone pair have moved in to the double bond (giving the N a + charge), and electrons of the C=O double bond have moved out to the O (giving it a - charge).
The resonance system shown in Fig 2 is based on measurements of the properties of amides. That is, detailed study of amides shows that the properties are better explained by Fig 2 than by Fig 1. As examples:
The bond length measured for amides is about half way between that typical for C-N single bonds and C=N double bonds. This is easily explained by the resonance system shown in Fig 2, which suggests that the actual bond between C and N is about a 1 1/2 bond.
A double bonded structure, or a structure with a substantial contribution of double bonding, would be expected to be planar, without free rotation about the C-N bond. This fits with observation.
The left hand structure in Fig 2 might look like it would accept an H+ on the N, thus acting as a base. However, the right hand structure has no lone pair, and even has a positive charge on the N. These features argue against the N being basic. A resonance system with a substantial contribution of the right hand structure would not be expected to be basic.
| All feedback encouraged. Please e-mail me any comments/corrections/suggestions. See Contact information, below. |
Home page for Musings (newsletter -- current science) Intro Chem (X11) Organic/Biochem (X402) Biotechnology in the News (BITN) Molecular Biology
Contact information Site home page
Last update: March 1, 2019