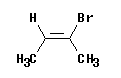Home
> Org/Bio Chem
> The E-Z system for naming alkenes; examples of using the CIP rules
| Contact
Introductory Organic and Biochemistry
The E-Z system for naming alkenes;
examples of using the CIP rules
A previous X402 textbook, Bettelheim 6/e, did not cover the E-Z system. Therefore, I wrote this page as a supplement to introduce the topic for those who wanted to go beyond the book. The new X402 book, Ouellette 2/e, covers the E-Z system fine, in Ch 4. Students taking my X402 with the Ouellette book are responsible for the E-Z system, as covered in the book. I have since added more material, especially more examples, here, to supplement Ouellette.
Textbook references on this page are to the previous X402 book, Bettelheim et al, 6/e -- except as noted. Students using that book will probably find it easier to learn the E-Z system for naming alkene isomers after covering the R-S system for chiral compounds in Ch 15.
On my page RasMol - An Introductory Guide, much use is made of Dave Woodcock's collection of molecular structure files at Okanagan University College. Most of the alkene files there are labeled in E-Z nomenclature.
Contents:
Introduction to the E,Z system
E,Z will work -- even when cis,trans fails
E,Z will work -- but may not seem to agree with cis,trans
Multiple double bonds. How to show E,Z for more than one double bond in a molecule.
The double-bond rule in determining priorities. How a double bond in the attached group helps determine E,Z.
The "first point of difference" rule. How do you really compare the three atoms on one C with the three atoms on another C?
Bottom of page; return links and contact information
Introduction to the E,Z system
The traditional system for naming the geometric isomers of an alkene, in which the same groups are arranged differently, is to name them as cis or trans. However, it is easy to find examples where the cis-trans system is not easily applied. IUPAC has a more complete system for naming alkene isomers. The Bettelheim textbook does not introduce it, but it is actually rather straightforward.
In Ch 15 Sect 3 Bettelheim presents the R-S system for naming stereoisomers that differ only in the arrangement of groups around a chiral center. The R-S system is based on a set of "priority rules", which allow you to rank any groups. These rules are summarized in Bettelheim 6/e Table 15.1, p 359. (They are in Ouellette 2/e, pp 108-9.)
The priority rules are often called the Cahn-Ingold-Prelog (CIP) rules, after the chemists who developed the system.
The rigorous IUPAC system for naming alkene isomers, called the E-Z system, is based on the same priority rules.
The general strategy of the E-Z system is to analyze the two groups at each end of the double bond. At each end, rank the two groups, using the CIP priority rules, discussed in Ch 15. Then, see whether the higher priority group at one end of the double bond and the higher priority group at the other end of the double bond are on the same side (Z, from German zusammen = together) or on opposite sides (E, from German entgegen = opposite) of the double bond.
Example...
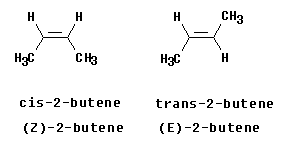 The Figure at the left shows the two isomers of 2-butene. You should recognize them as cis and trans. Let's analyze them to see whether they are E or Z. Start with the left hand structure (the cis isomer). On C2 (the left end of the double bond), the two atoms attached to the double bond are C and H. By the CIP priority rules, C is higher priority than H (higher atomic number). Now look at C3 (the right end of the double bond). Similarly, the atoms are C and H, with C being higher priority. We see that the higher priority group is "down" at C2 and "down" at C3. Since the two priority groups are both on the same side of the double bond ("down", in this case), they are zusammen = together. Therefore, this is (Z)-2-butene.
The Figure at the left shows the two isomers of 2-butene. You should recognize them as cis and trans. Let's analyze them to see whether they are E or Z. Start with the left hand structure (the cis isomer). On C2 (the left end of the double bond), the two atoms attached to the double bond are C and H. By the CIP priority rules, C is higher priority than H (higher atomic number). Now look at C3 (the right end of the double bond). Similarly, the atoms are C and H, with C being higher priority. We see that the higher priority group is "down" at C2 and "down" at C3. Since the two priority groups are both on the same side of the double bond ("down", in this case), they are zusammen = together. Therefore, this is (Z)-2-butene.
Now look at the right hand structure (the trans isomer). In this case, the priority group is "down" on the left end of the double bond and "up" on the right end of the double bond. Since the two priority groups are on opposite sides of the double bond, they are entgegen = opposite. Therefore, this is (E)-2-butene.
top of page
E,Z will work -- even when cis,trans fails
In simple cases, such as 2-butene, Z corresponds to cis and E to trans. However, that is not a rule. This section and the following one illustrate some idiosyncrasies that happen when you try to compare the two systems.
The real advantage of the E-Z system is that it will always work. In contrast, the cis-trans system breaks down with many ambiguous cases.
Example...
The following figure shows two isomers of an alkene with four different groups on the double bond, 1-bromo-2-chloro-2-fluoro-1-iodoethene.
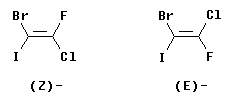 It should be apparent that the two structures shown are distinct chemicals. However, it is impossible to name them as cis or trans. On the other hand, the E-Z system works fine... Consider the left hand structure. On C1 (the left end of the double bond), the two atoms attached to the double bond are Br and I. By the CIP priority rules, I is higher priority than Br (higher atomic number). Now look at C2. The atoms are Cl and F, with Cl being higher priority. We see that the higher priority group is "down" at C1 and "down" at C2. Since the two priority groups are both on the same side of the double bond ("down", in this case), they are zusammen = together. Therefore, this is the (Z) isomer. Similarly, the right hand structure is (E).
It should be apparent that the two structures shown are distinct chemicals. However, it is impossible to name them as cis or trans. On the other hand, the E-Z system works fine... Consider the left hand structure. On C1 (the left end of the double bond), the two atoms attached to the double bond are Br and I. By the CIP priority rules, I is higher priority than Br (higher atomic number). Now look at C2. The atoms are Cl and F, with Cl being higher priority. We see that the higher priority group is "down" at C1 and "down" at C2. Since the two priority groups are both on the same side of the double bond ("down", in this case), they are zusammen = together. Therefore, this is the (Z) isomer. Similarly, the right hand structure is (E).
top of page
E,Z will work -- but may not seem to agree with cis,trans
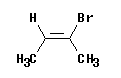
|
Consider the molecule shown at the left.
This is 2-bromo-2-butene -- ignoring the geometric isomerism for now.
|
Cis or trans? This molecule is clearly cis. The two methyl groups are on the same side. More rigorously, the "parent chain" is cis.
E or Z? There is a methyl at each end of the double bond. On the left, the methyl is the high priority group -- because the other group is -H. On the right, the methyl is the low priority group -- because the other group is -Br. That is, the high priority groups are -CH3 (left) and -Br (right). Thus the two priority groups are on opposite sides = entgegen = E.
This example should convince you that cis and Z are not synonyms. Cis/trans and E,Z are determined by distinct criteria. There may seem to be a simple correspondence, but it is not a rule. Be sure to determine cis,trans or E,Z separately, as needed.
top of page
Multiple double bonds
If the compound contains more than one double bond, then each one is analyzed and declared to be E or Z.
Example...
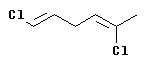
|
The configuration at the left hand double bond is E; at the right hand double bond it is Z. Thus this compound is (1E,4Z)-1,5-dichloro-1,4-hexadiene.
|
top of page
The double-bond rule in determining priorities
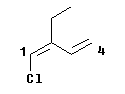
|
This section is intended as a clarification of Ouellette's explanation, p 108.
Consider the compound shown at the left.
|
This is 1-chloro-2-ethyl-1,3-butadiene -- ignoring, for the moment, the geometric isomerism.
There is no geometric isomerism at the second double bond, at 3-4, because it has 2 H at its far end.
What about the first double bond, at 1-2? On the left hand end, there is H and Cl; Cl is higher priority (by atomic number). On the right hand end, there is -CH2-CH3 (an ethyl group) and -CH=CH2 (a vinyl or ethenyl group). Both of these groups have C as the first atom, so we have a tie so far and must look further. What is attached to this first C? For the ethyl group, the first C is attached to C, H, and H. For the ethenyl group, the first C is attached to a C twice, so we count it twice; therefore that C is attached to C, C, H. CCH is higher than CHH; therefore, the ethenyl group is higher priority. Since the priority groups, Cl and ethenyl, are on the same side of the double bond, this is the Z-isomer; the compound is (Z)-1-chloro-2-ethyl-1,3-butadiene.
As noted, this section is intended as a clarification of Ouellette's explanation. At the bottom of p 108 he shows and analyzes a double bond group. The group he shows is the attached group whose priority is being analyzed; he does not show the double bond to which it is attached, whose E,Z-ness is being analyzed. Some students have found that unclear. In my example here, I show both explicitly. The 1-2 double bond is being analyzed for E,Z; the 3-4 double bond is being analyzed for priority as part of that.
top of page
The "first point of difference" rule
Which is higher priority, by the CIP rules: a C with an O and 2 H attached to it or a C with three C? (Ouellette raises this in Exercise 6.9, part c.) The first C has one atom of high priority but also two atoms of low priority. How do these "balance out"? Answering this requires a clear understanding of how the ranking is done. The simple answer is that the first point of difference is what matters; the O wins.
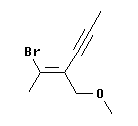
| To illustrate this, consider the molecule at the left. Is the double bond here E or Z?
At the left end of the double bond, Br > H. But the right end of the double bond requires a careful analysis.
|
At the right hand end, the first atom attached to the double bond is a C at each position. A tie, so we look at what is attached to this first C. For the upper C, it is CCC (since the triple bond counts three times). For the lower C, it is OHH -- listed in order from high priority atom to low. OHH is higher priority than CCC, because of the first atom in the list. That is, the O of the lower group beats the C of the upper group. In other words, the O is the highest priority atom of any in this comparison; thus the O "wins".
Therefore, the high priority groups are "up" on the left end (the -Br) and "down" on the right end (the -CH2-O-CH3). This means that the isomer shown is opposite = entgegen = E.
And what is the name? The "name" feature of ChemSketch says it is (2E)-2-(1-bromoethylidene)pent-3-ynyl methyl ether. That name is well beyond our course! However, you may be able to figure out much of it. I will try to explain it, upon request.
|
All feedback encouraged. Please e-mail me any comments/corrections/suggestions. See Contact information, below.
|
Top of page
Organic/Biochem (X402) home page
Organic/Biochem (X402) Internet resources
Contact information
Site home page
Last update: February 09, 2019
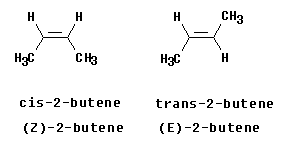 The Figure at the left shows the two isomers of 2-butene. You should recognize them as cis and trans. Let's analyze them to see whether they are E or Z. Start with the left hand structure (the cis isomer). On C2 (the left end of the double bond), the two atoms attached to the double bond are C and H. By the CIP priority rules, C is higher priority than H (higher atomic number). Now look at C3 (the right end of the double bond). Similarly, the atoms are C and H, with C being higher priority. We see that the higher priority group is "down" at C2 and "down" at C3. Since the two priority groups are both on the same side of the double bond ("down", in this case), they are zusammen = together. Therefore, this is (Z)-2-butene.
The Figure at the left shows the two isomers of 2-butene. You should recognize them as cis and trans. Let's analyze them to see whether they are E or Z. Start with the left hand structure (the cis isomer). On C2 (the left end of the double bond), the two atoms attached to the double bond are C and H. By the CIP priority rules, C is higher priority than H (higher atomic number). Now look at C3 (the right end of the double bond). Similarly, the atoms are C and H, with C being higher priority. We see that the higher priority group is "down" at C2 and "down" at C3. Since the two priority groups are both on the same side of the double bond ("down", in this case), they are zusammen = together. Therefore, this is (Z)-2-butene.
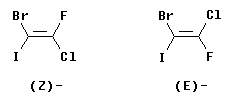 It should be apparent that the two structures shown are distinct chemicals. However, it is impossible to name them as cis or trans. On the other hand, the E-Z system works fine... Consider the left hand structure. On C1 (the left end of the double bond), the two atoms attached to the double bond are Br and I. By the CIP priority rules, I is higher priority than Br (higher atomic number). Now look at C2. The atoms are Cl and F, with Cl being higher priority. We see that the higher priority group is "down" at C1 and "down" at C2. Since the two priority groups are both on the same side of the double bond ("down", in this case), they are zusammen = together. Therefore, this is the (Z) isomer. Similarly, the right hand structure is (E).
It should be apparent that the two structures shown are distinct chemicals. However, it is impossible to name them as cis or trans. On the other hand, the E-Z system works fine... Consider the left hand structure. On C1 (the left end of the double bond), the two atoms attached to the double bond are Br and I. By the CIP priority rules, I is higher priority than Br (higher atomic number). Now look at C2. The atoms are Cl and F, with Cl being higher priority. We see that the higher priority group is "down" at C1 and "down" at C2. Since the two priority groups are both on the same side of the double bond ("down", in this case), they are zusammen = together. Therefore, this is the (Z) isomer. Similarly, the right hand structure is (E).